From AFFF to FF3 : Impact — Part 4
As detailed previously in first three parts of this series of articles, we have seen that the perfluorochemicals have a long history spanning more than 50 years and are far more complex than just considering PFOS, PFHxS and PFOA. Rather there are literally thousands of perfluorochemicals used in industry and commerce and focusing solely on a few named structures is unhelpful and a form of ‘tunnel vision’.

Currently there have been strong arguments put forward to treat PFAS as a class of chemical compounds, rather than as individual compounds each requiring the assessment of risk [Kwiatkowski et al (2020)], as a means of countering the disadvantages of concentrating on a few named PFAS. In seeking to regulate PFAS use the concept of ‘essential uses’ has been proposed by the Cousins group [Roy et al. (2022); Figuiére et al. (2023)].
Independent academic and regulatory interest in this class of compounds was virtually non-existent, with some notable exceptions, until the 3M Company announced in May 2000 that it was phasing-out all PFOS-based chemistry replacing it with PFBS chemistry (perfluorobutane sulfonic acid, the C4 homologue of PFOS). A notable exception which was highly relevant to understanding environmental contamination caused by AFFF foams was work published by Jennifer Field and her colleagues at Oregon State University around the same time or shortly after 3M announced its withdrawal from PFOS chemistry and foam manufacture [Moody et al. (2000); Schultz et al. (2004)].
Investigative activity concerning this class of compounds started shortly after the 3M announcement in May 2000. At first most of the contributions were from the industry itself or from groups funded by the industry. It was in the late 2000s that the independent scientific community realized that there were far more that needed investigating about the impact of PFAS both on human health and the environment, with the result that the number of peer-reviewed publications increased exponentially..
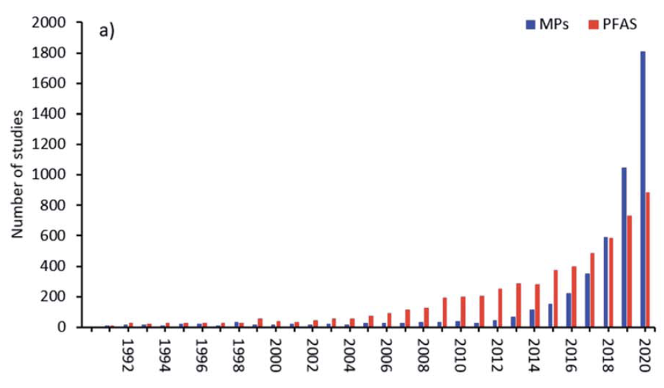
Publications concerned with PFAS in the run-up to May 2000 were only in the range of 5-10 per year. After May 2000 these increased to around 50 per year and have increased exponentially since now reaching ~1000/year, frequently associated with a parallel increase in articles on microplastics [Bakhshoodeh and Santos (2022)], from whom Figure 2(b) is taken.
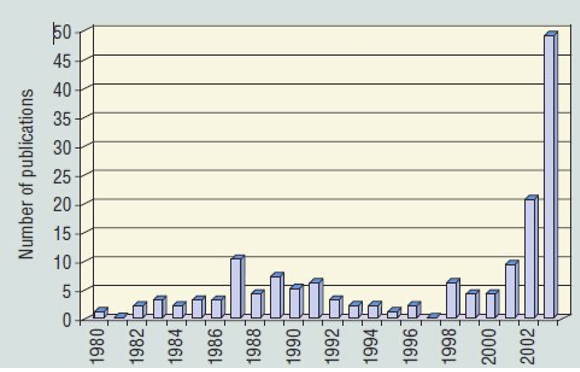
Published studies on the scale of environmental by PFOS and PFOA were scarce before 2001, until Giesy and Kannan [Giesy and Kannan (2001) and subsequent papers] reported global presence of PFOS in wildlife, with Hansen et al. finding PFOS and PFOA downstream from a manufacturing facility on the Tennessee River [Hansen et al. (2002)], as well as in biological matrices using mass spectrometry [(Hansen et al. (2001)].
Although contamination of human blood samples with organofluorine chemicals not present in stored blood taken before fluorochemical manufacturing began had been reported some years earlier since the 1960s [Taves, D.R. (1966, 1968)] with Guy et al. (1976) reporting the presence of fluorochemicals in human plasma using NMR spectroscopy, tentatively identifying PFOA as the fluorochemical used in Scotchgard® as blood contaminant [Guy, W.S. et al. (1976)]. Although there was initial confusion whether this fluorochemical was indeed PFOA or PFOS, as a result of obfuscation and a refusal to identify PFOS by 3M, it was not until 25 years later that Hansen et al. (2001) reported that samples of human plasma contained PFOS (average 28.4 ng/ml), PFHxS (average 6.6 ng/ml) and PFOA (average 6.4 ng/ml), confirming Guy and Taves findings.
A lawsuit filed in 2010 by the Attorney General of the State of Minnesota against the 3M Company revealed that the company knew these chemicals were accumulated in human blood for more than 40 years and were toxic [Lerner (2018); Swanson (2019)].
Until about 2004 the scientific literature was dominated by papers from authors working directly for 3M or funded by the industry. Post 2004 there has been an exponential explosion of independent published work concerned with PFOS, PFOA and other PFAS, to the extent that these organofluorine compounds have been labelled ‘emerging contaminants’. PFAS have emerged as contaminants of concern for at least a decade. The current situation is that although we now know a considerable amount about the environmental distribution, fate and toxicity to biota of PFAS, it is the technology for the remediation and disposal of these highly persistent materials that should be regarded as ‘emerging science’.
As said before, we are not only talking of a few substances, but about a whole family of at least 6000 different perfluorocompounds which have been classified for at least one environmental, human health and/or physicochemical endpoint in the ECHA database. At the UN Stockholm Convention Persistent Organic Pollutants Review Committee meeting in Rome (POPRC-14) an indicative list of PFOA-related substances contained 4,700 entries.
Human health endpoints are considered of major importance for long-term exposure: carcinogenicity ©; mutagenicity (M0; reproductive toxicity (R); lactation effects (L) and specific organ toxicity (STOT). 388 PFAS have at least one of these five endpoints, of which 44 are registered in harmonized classification.
With regards to environmental hazards, 1129 PFAS have been registered by self-classification; most of them counting as both (M) mobile and/or very persistent (vP).
Under the EU chemicals legislation (REACH and ECHA) the risks posed by PFAS are classified using the PBT system indicating persistence (P or vP), bio-accumualation (B or vB), and toxicity (T). Recent proposals from the German Federal Environment Agency (UBA) also stress the importance of mobility (M or vM) especially for PFAS in view of their vP and vM properties [Arp et al. (2023)]. Chemicals may also be identified as Substances of Very High Concern (SVHC) under REACH EC1907/2006 if they have serious and often irreversible effects on human health or the environment. A recent example is perfluorononanoic acid or PFNA, a PFAS that is becoming more commonly found as a contaminant. In Europe there has been far more concern with individual components of the PBT classification whereas in the United States persistence on its own with accompanying toxicity is seen as less of a problem.
Persistence
PFAS or their perfluorinated degradation products are among the most chemically and physically stable organic compounds known. Their perfluorinated carbon chains resist environmental and metabolic degradation due to the very stable C-F bonds. For example, tetrafluoromethane, CF4 and the simple perfluorocarbon, has an estimated atmospheric half-life of ~50,000 years with a high global warming potential (GW) [Mühle et al.(2010)].
Commercially available perfluoro compounds are designed to degrade rapidly once released into the environment yielding PFCAs, PFECAs and PFSAs. Unfortunately, this has led in the past to totally spurious and misleading claims made by the industry especially in the United States that these materials are ‘biodegradable’ based on degradation of the non-fluorinated functional group and the OECD rule that a substance if ‘readily biodegradable’ if degradation reaches 60% (OECD 301B, D and F) or 70% (OECD301A and E) within 28 days. This does not mean that the PFAS, for example in firefighting AFFF, is completely biodegradable as the COD method using acid dichromate for the oxidation measurement to give the 100% level totally fails to account for any perfluorinated material present. Regrettably end-users have often assumed or been led to believe by salesmen, and perpetrated as a myth by certain manufacturer [Swanson, 2019], that ‘readily biodegradable’ means total degradation. As pointed out earlier it makes sense, however, to group all PFAS as non-degradable according to their stable degradation end-products.
Breakdown of the precursors often leads to the formation of PFAS intermediates and ultimate degradation products with increased mobility in water and/or air via oxidative chemical and biochemical degradation processes in the environment.
Lifetimes of the PFAS in the environment greatly exceed the criteria for very persistent (vP) substances in Annex XIII to REACH. If PFAS do degrade, they do it so slowly that it is not observable in standard tests. The extreme persistence of PFAS and their continued use leads to sustained exposure and increasing concentrations in all environment compartments. PFASs will remain in the environment for very long time, even if releases are minimized. Increasing or legacy contamination of the environment will increase the likelihood that known and unknown effects will occur on a generational timescale. This should invoke the application of the Precautionary Principle [Rio 1992; Preston, 2017] to any further dispersive use of PFAS.
Scientists pointed out in the Helsingør Statement on PFASs [Scheringer et al., 2014] as well as in the Madrid statement [Blum et al., 2015] that very high persistence on its own presents a problem and have named this the “P-sufficient approach” to regulatory action. Persistence alone justified the regulation of PFAS as a class in California [Balan et al., 2021].
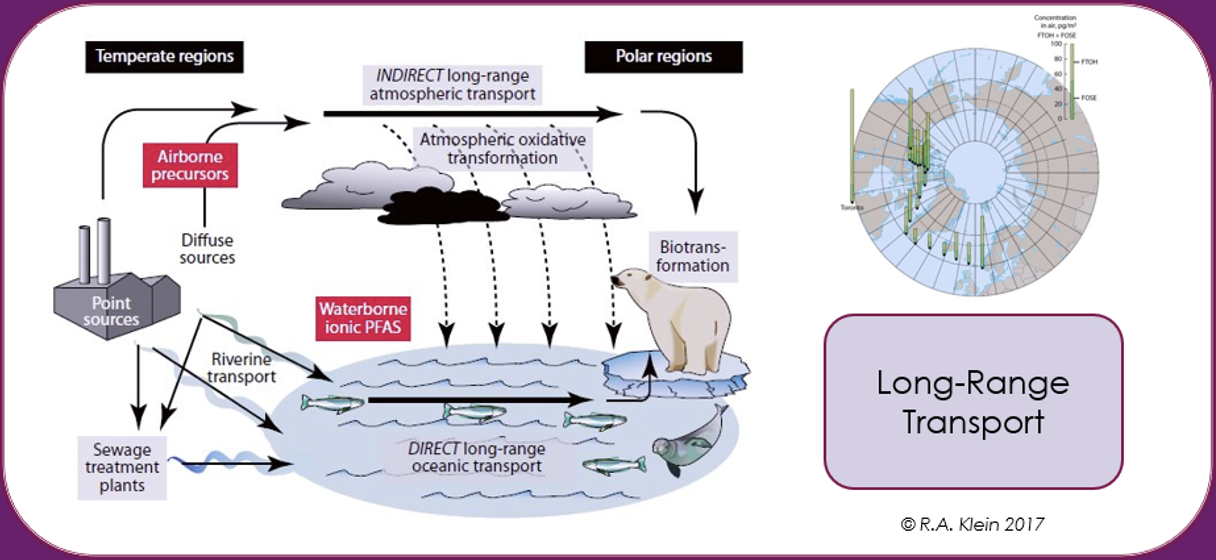
Long range transport potential (LRTP)
PFAS can be transported by air, water and matrices to which they are adsorbed or absorbed, such as dust, sediments, marine aerosols, oceanic currents, atmospheric, migratory animals, or through matrices in which they are included as additive such as microplastics. Because of their remarkable resistance to degradation, this leads to global dispersion of PFAS over long distances from the point of release. It has been estimated, for example, that for volatile PFAS such as fluorotelomer alcohols in the upper atmosphere that global circulation times can be short as 7-10 days. The historical spread through LRTP of PFAS through deposition and contamination in the Arctic is well documented [Wilson et al. (AMAP) Secretariat, 2017].
The Inuit population in the Arctic has been reported as being some of the most contaminated humans on the planet as the PFAS concentrations in their blood is much higher than the average value for the general population. Being so far removed from any industrial source of PFAS, this contamination has been attributed mainly to their diet, which based on fish, polar bear and seal meat, with an impact on the immune response [Sonne et al., 2023].
Thus, PFAS contamination of the environment and biota is not limited geographically just to the source of the pollution but becomes widespread on a global scale due to dispersive use such as in firefighting foams, poor waste disposal techniques or industrial production, compounded by long-range transport in the atmosphere and oceans.
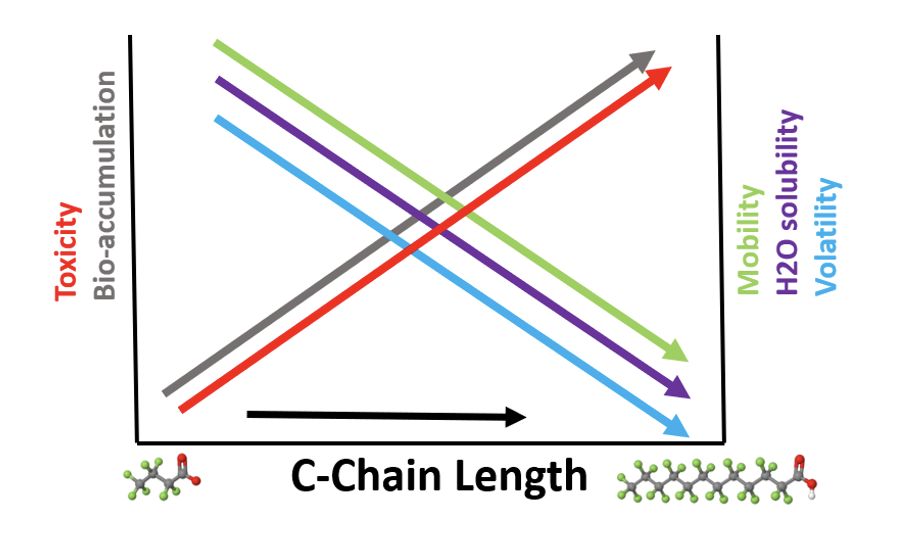
Mobility
It is generally considered that substances with moderate to high solubility in water associated with low adsorption potential have a high mobility in the aqueous environment. Various studies have shown that PFAS have a different behavior depending strongly on carbon chain length and on functionality.
As shown in the figure below shorter chain-length PFAS are associated with higher environmental mobility, water solubility and volatility, as well as lower toxicity and bio-accumulation potential than longer chain-length PFAS. The combination of extreme persistence and high mobility in the aqueous compartment and soils, especially for the shorter chain PFAS such as PFPeS and PFBS, leads to contamination of drinking water aquifers and rivers as well as uptake into the food chain – fish, plants and livestock.
Accumulation in plants
A recent review article on exposure routes, bio-accumulation and toxic effects of PFASs on plants shows that bio-accumulation processes of PFASs in plants highly vary because of the complexity of PFAS chemistry [Li et al., 2022].
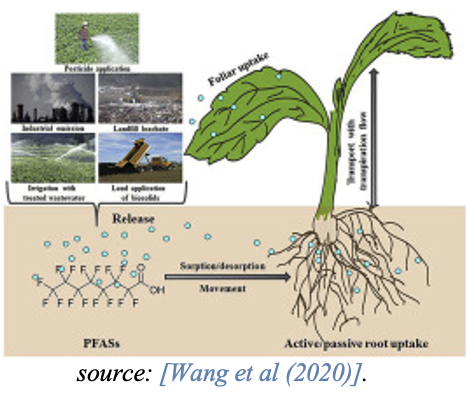
Whereas short-chain PFASs typically accumulate in above-ground plant parts such as leaves, long-chain PFASs accumulate in roots and show lower translocation factors to the above-ground plant parts. This is influenced by the higher water solubility, lower molecular size and lower hydrophobicity of the short-chain PFASs. Studies also indicate that the short-chain PFCA are more effectively taken up by plants than the long-chain PFCA [Felizeter et al., 2014; Yoo et al., 2011].
Consumption of plant material, e.g., grains and vegetables either as roots or above ground plant parts such as leaves or stems, function as a source of PFAS for both humans and animals.
Bio-accumulation and Bio-magnification (tropic magnification)
Under REACH, C11-C14 PFCAs and C6-PFSA have been shown to fulfil the vB-criterion and C8-C10-PFCA the B criterion.
Studies with mammalian species show that PFASs are readily absorbed and distributed across various tissues and that some PFAS (particularly the long-chain PFAS) have long half-lives in organisms, especially in humans where half-lives are of the order of years. Studies show that PFAS binding to albumin and transporter proteins efficiently distributes PFASs into different tissues, and enhances passage across both brain and placental barriers, with transfer to neonates via breast milk. Because of their hydrophobic and oleophobic properties PFAS do not follow typical accumulation patterns, like partitioning into adipose tissue, but rather bind and accumulate in protein-rich organs like liver.
PFASs accumulate more in air-breathing organisms as compared to gill breathing- organisms, because unlike the latter, air-breathers cannot readily eliminate PFAS by passive diffusion. Thus, established methods of bio-accumulation testing in aquatic organisms do not function adequately as methodology for PFAS bio-accumulation assessments in air-breathing species such as man, Unfortunately, laboratory bio-accumulation data are very limited for air-breathers.
Short-chain PFASs are more readily excreted by urinary excretion in air-breathing organisms and tend to be less bio-accumulative, while bio-accumulation potential usually increases with perfluoroalkyl chain length. In general, BCFs and BAFs of PFASs with 8 or more carbons increase uniformly with increasing number of carbons in the alkyl chain, with highest bio-accumulation potential seen for compounds with 12 to 14 carbon-chain length.
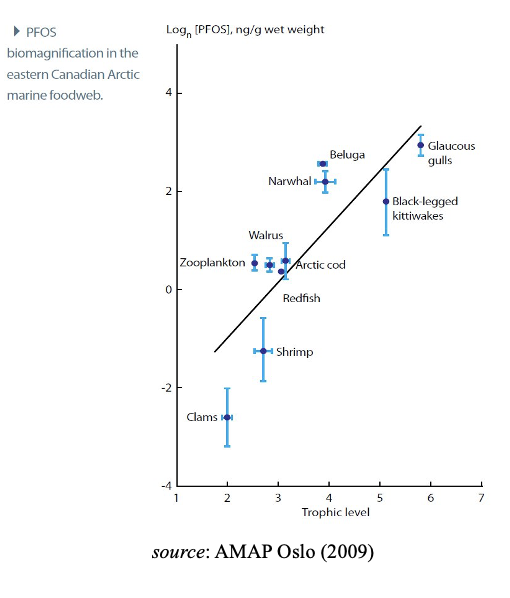
Due to these properties, many PFASs accumulate in air-breathers, and long- chain PFASs bio-magnify in marine and fresh-water food webs, reaching high levels in top predators including humans and vulnerable species. This increase in contamination seen as one ascends the food chain is known as trophic magnification and is well established for aquatic species and predators that feed off them. It is noted that consequently this may negatively affect the recommendations related to consumption of meat and/or entrails of certain animals. A a top marine predator guillemot eggs are particularly high in PFAS.Field studies on long- and short-chain PFASs that can be analytically distinguished demonstrate that PFAS
(primarily PFBA, PFBS, PFHpA, PFHxA, PFHxS, PFOS, FOSA, 6:2 FTOH, F-53B, 6:2 Cl-PFESA, TFA, and C9-C11 PFCAs) are found globally throughout the environment in mammals, birds, fish and other vertebrates. In conclusion, and considering the increasing lines of evidence from modelling, laboratory and monitoring studies, there is a justifiable rising level of concern for a subset of PFAS being bio-accumulative while large uncertainties remain for the majority of compounds due to lack of data.
Effects on human health
A vast amount of literature has been published on the health effects of PFAS, especially for PFOA and PFOS. In humans, many perfluoroalkyl acids (PFAA) are readily absorbed by inhalation or ingestion orally, while less is known regarding absorption after dermal exposure. Many PFAA bind to proteins and are thus distributed to protein-rich tissues including liver, kidneys, and blood. Estimated human elimination half-lives for PFAAs range from a few days (PFBA) and months (PFHxA, PFBS) to a few (2-8) years (PFOA, PFNA, PFDA, PFHxS, PFOS), or to >10 years for PFUnDA. Half-lives are much shorter in rodents than in humans and differences in half-lives between sexes is often observed. Consequently, the observed toxicity in rodents underestimates the toxicity to humans. PFAA are mainly excreted via urine and faeces and thus are released to the environment. PFAA have a marked potential for bio-accumulation in humans as shown by the long half-lives and protein-binding.
The European Food Standards Agency (EFSA) extensively reviewed the epidemiological evidence for association between PFAS exposure and adverse effects in humans [EFSA, 2018; EFSA, 2020]. EFSA concluded that increased serum levels of various PFCA and PFSA provoked a reduction in the immune response to vaccination [Grandjean, 2012], increased propensity of infections, increased serum cholesterol, increased serum alanine transferase (ALT) and reduced birth weight. The association with immune effects was considered the most sensitive endpoint in humans (supported by data from experimental animals) and based on this EFSA has established a Tolerable Weekly Intake (TWI) of 4.4 ng/kg body weight/week for the sum of PFOA, PFOS, PFNA and PFHxS [EFSA, 2020].
Experimental animal studies across different groups of PFAS demonstrate that liver, kidney, thyroid, the immune system, and reproduction are major targets for PFAAs’ toxicity. In rat studies, the most consistent effects included enlarged liver, hepatocellular hypertrophy, increased serum ALT, increased kidney weight, reprotoxicity, effects on the lymph system, and decreased serum thyroid hormone levels. In particular liver effects have been observed for most PFAA for which animal studies are available. For PFOS, PFOA, PFNA, and PFDA and their salts this has resulted in harmonized classifications for carcinogenicity (Carc. 2), reproductive toxicity (Repr. 1B), lactation effects (Lact.) and specific target organ toxicity – repeated exposure (STOT RE 1, except for PFDA).
Cumulative effects of co-occurring PFAS
Many different PFAS co-occur in the environment, drinking water, food, and in human blood. Many PFAS exhibit similar effects, such as effects on the liver, kidney, thyroid, serum lipids, and immune system. Accordingly, an assessment of hazards and risks taking into account such combined exposure would reflect exposure conditions more realistically than single compound assessments.
Due to the immense number of PFAS and the lack of toxicological data for the vast majority of them, a combined assessment for all PFASs is unattainable. It is emphasized at this point that combined exposure to different PFASs affecting the same target organs may result in combined more than additive effects, i.e., synergism, making the exceeding of thresholds or limit values more likely than for assessment for individual substances on their own.
CONCLUSION
PFAS are very Persistent (vP) and many are also very Bioaccumulative (vB). Long-range transport processes (LRTP) results in planetary contamination including remote regions such as the Arctic.
Cousins et al. have recently introduced the concept of having already exceeded the ‘planetary boundary’ indicating that global environmental concentrations for PFAS already exceed tolerable sustainable levels [Cousins et al., 2022]. This should be treated as a warning against continuing use and release of PFAS to the environment, especially from dispersive applications such as AFFF firefighting foam.
The permanent presence of PFAS in human blood indicates the level of continued exposure of the general population. PFAS are present in drinking water and in food stocks. Hundreds of scientific studies have highlighted the long-term toxicity of PFAS, effecting the liver, kidneys, thyroid and immune system. The ubiquitous presence of PFAS in human blood and other species worldwide highlights the dangers associated with continued manufacturing and use in industrial and consumer products of extremely persistent organic pollutants which are totally anthropogenic in origin, not occurring naturally.
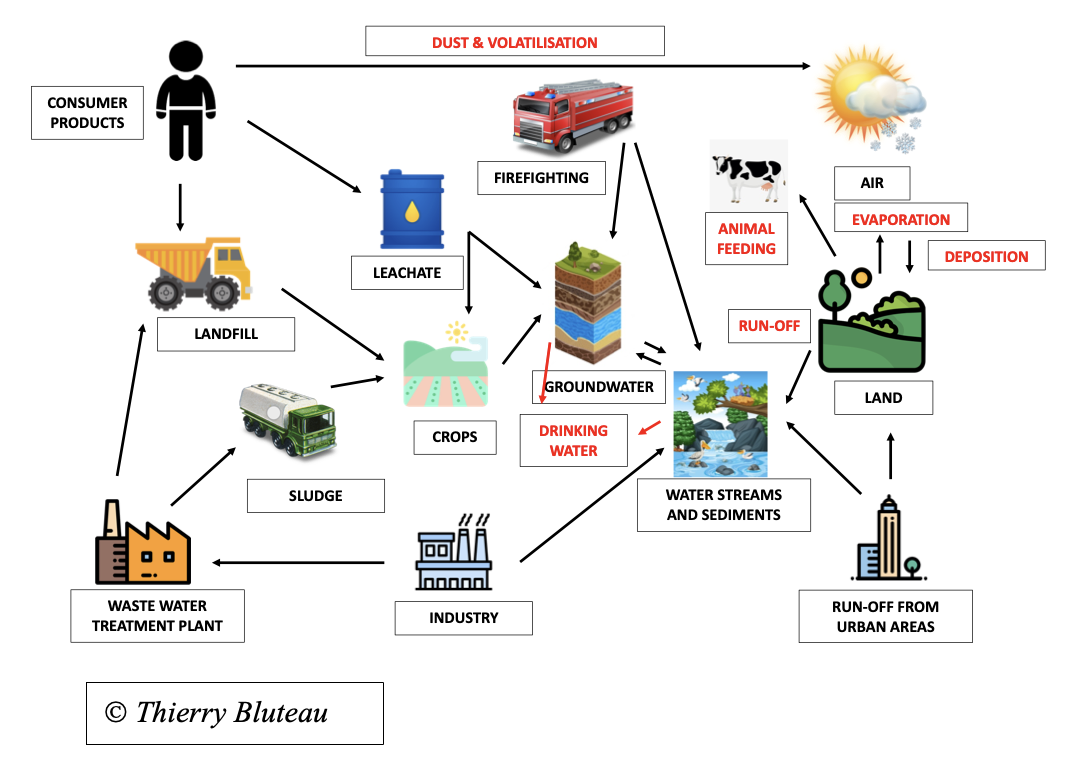
This schema summarizes all possibilities for PFAS to contaminate the environment and living beings including humans.
References
Arp, H.P.M., Hale, S.E., Borchers, U., Valkov, V., Wiegand, L., Zahn, D., Neuwald, I., Nödler, K., and Scheurer, M. (2023) A prioritization framework for PMT/vPvM Substances under REACH for registrants, regulators, researchers and the water sector. German Environment Agency (Umweltbundesamt) Report 22/2023 pp. 1-238.
Bakhshoodeh, R., and Santos, R.M. (2022) Comparative bibliometric trends of microplastics and perfluoroalkyl and polyfluoroalkyl substances: how these hot environmental remediation research topics developed over time. RSC Advances 12, 4973-4987.
Bălan, .A., ChanderMathrani, V., Fengmao Guo, D., and Algazi1, A.M. (2021) Regulating PFAS as a Chemical Class under the California Safer Consumer Products Program. Environ. Health Perspect, 129 (20) February 025001, 1-9.
Blum, A., Balan, S.A., Scheringer, M., Trier, X., Goldenman, G., Cousins, I.T., Diamond, M., Fletcher, T., Higgins, C.P., Lindeman, A.E., Peaslee, G., de Voogt, P., Wang, Z., and Weber, R. (2015) The Madrid Statement on Poly- and Perfluoroalkyl Substances (PFASs). Environ. Health Perspect. 123 (5) May A107-A11.
Cousins, I.T., Johansson, J.H., Salter, M.E., Sha, B. and Scheringer, M. (2022) Outside the Safe Operating Space of a New Planetary Boundary for Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Technol. 2022, 56, 11172−11179.
European Food Standards Agency (EFSA) (2018) Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA Journal 16(12), 5194.
European Food Standards Agency (EFSA) (2020) Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal 18(9), 6223.
Felizeter, S., McLachlan, M.S., and de Voogt, P. (2014) Root uptake and translocation of perfluorinated alkyl acids by three hydroponically grown crops. J. Agric. Food Chem. 62 (15), 3334-42.
Figuiére, R., Borchert, F., Cousins, I.T., and Ågerstrand, M. (2023) The essential-use concept: a valuable tool to guide decision-making on applications for authorization under REACH? Environmental Sciences Europe 35 (5) 1-12.
Giesy, J.P., and Kannan, K. (2001) Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 35(7) 1339-1342.
Grandjean, P., Andersen, E.W., Budtz-Jørgensen, E., Nielsen, F., Mølbak, K., Weihe, P., and Heilmann, C. (2012) Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307(4), 391-7.
Guy, W.S., Taves, D.R., Brey, W.S. (1976) Fluorocompounds in Human Plasma: Prevalence and Characterization. Biochemistry Involving Carbon-Fluorine Bonds, ACS Symposium. 117-134
Hansen, K.J., Clemen, L.A., Eellefson, M.E., and Johnson, H.O. (2001) Compound-specific quantitative characterization of organic fluorochemicals in biological matrices. Environ. Sci. Technol. 35(4) 766-770.
Hansen K.J., Johnson, H.O., Eldridge, J.S., Butenhoff, J.L., and Dick, L.A. (2002) Quantitative Characterization of Trace Levels of PFOS and PFOA in the Tennessee River. Environ. Sci. Technol. 36(8) 1681-1685.
Kwiatkowski, C.F., Andrews, D.Q., Birnbaum, L.S., Bruton, T.A., DeWitt, J.C., Knappe, D.R.U., Maffini, M.V., Miller, M.F., Pelch, K.E., Reade,A., Soehl, A., Trier, X., Venier, M., Wagner, C.C., Wang, Z., and Blum, A. (2020) Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol., 7, 532−543.
Lerner, S. (2018)] 3M knew about the Dangers of PFOS and PFOA Decades Ago, Internal Documents Show. The Intercept July 18 2018.
Li, J., Sun, J., and Li, P. (2022) Exposure routes, bioaccumulation and toxic effects of per- and polyfluoroalkyl substances (PFASs) on plants: A critical review,. Environ. International 158, 106891.
Moody, C.A., and Field, J.A. (2000) Perfluorinated Surfactants and the Environmental Implications of Their Use in Fire-Fighting Foams. Environ, Sci. Technol. 34(18), 3864-3869.
Mühle, J., Ganesan, A.L. , Miller, B.R, Salameh, P.K., Harth, C.M., Greally, B.R.,, Rigby, M., Porter, L.W., Steele, L.P., Trudinger, C.M.,, Krummel, P.B., O’Doherty, S.,, Fraser, P.J., Simmonds, P.G., Prinn, R.G.,, and Weiss, R.F.. (2010) Perfluorocarbons in the global atmosphere: tetra-fluoromethane, hexafluoroethane, and octafluoropropane. Atmos. Chem. Phys. 10, 5145–5164.
Preston, B.A. (2017) The Judicial Development of the Precautionary Principle. Queenslnad Government Environmental Management of Firefighting Foam Policy Implementation Seminar, Brisbane 21 Febraury 2017. pp. pp.1`-26. < https://lec.nsw.gov.au >.
Rio Convention (1992) UN Conference on Environment and Development, Rio de Janiero, Brazil, 3-14 June 1992.
Roy, M.A., Cousins, I.T., Harriman, H., Scheringer, M., Tickner. J.A., and Wang, Z. (2022) Combined Application of the Essential-Use and Functional Substitution Concepts: Accelerating Safer Alternatives Environ. Sci. Technol. 56, 9842−9846.
Scheringer, M., Trier, X., Cousins, I.T., de Voogt, P., Fletcher, T., Wang, Z., and Webster, T.F. (2014) Helsingør Statement on poly- and perfluorinated alkyl substances (PFASs). Chemosphere 114 (2014) 337–339.
Schultz, M.M., Barofsky, D.F., and Field, J.A. (2004) Quantitative Determination of Fluorotelomer Sulfonates in Groundwater by LC MS/MS. Environ. Sci. Technol. 38 (6) 1828-1835.
Sonne, C., Desforges, J.-P., Bossi, R., and Long, M. (2023) Assessment of Exposure to Perfluorinated Industrial Substances and Risk of Immune Suppression in Greenland and its Global Context: a Mixed-Methods Study. Lancet Planetary Health 7 (7), E570-E579, July 2023.
Swanson, L. (2019) Testimony of Lori Swanson Former Minnesota Attorney General before the Committee on Oversight and Reform Subcommittee on Environment United States House of Representatives. September 10, 2019. pp. 1-66.
Taves, D.R. (1966) Normal human serum fluoride concentrations. Nature 211, 192-193.
Taves, D.R. (1968) Evidence that there are Two Forms of Fluoride in Human Serum. Nature 217, 1050-1051.
Wang, W., Rhodes, G., YU, X., and Li, H. (2020) Uptake and accumulation of per- and polyfluoroalkyl substances in plants. Chemosphere 261, 127584.
Wilson, S., Fuglestad, J., Larsen, J.-R., Pawlak, J., and Utne, I. (2017) (AMAP Secretariat). Chemicals of Emerging Arctic Concern. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway. xvi+353pp.
Yoo, H., Washington, J.W., Jenkins, T.M., and Ellington, J.J. (2011) Quantitative determination of perfluorochemicals and fluorotelomer alcohols in plants from biosolid-amended fields using LC/MS/MS and GC/MS. Environ. Sci. Technol. 45(19), 7985-90.
